GAMP Validation: Ensuring Compliance and Quality in Industrial Automation
Using Good Automated Manufacturing Practices (GAMP) to validate an automated system can be a daunting, arduous process. Validation requires meticulous attention to detail, comprehensive documentation, revision logging, and testing of each change prior to final acceptance. At Automation NTH, our deep understanding of this complex process allows us to provide our customers with validation support that meets their requirements and streamlines the validation process.
AUTOMATION NTH: DEPENDABLE SYSTEMS, STREAMLINED VALIDATION
Automation NTH creates advanced, reliable custom automation systems with clean, trackable documentation that accelerates the validation process. Our standard procedures are documented in our Quality Assurance Manual (QAM) and are complemented by our customizable validation options we provide to our customers.
During the proposal phase, we facilitate an open dialogue between Automation NTH’s Engineers and the customer’s team to understand the validation goals and how we can help. We then develop a scope of work with a deliverable list including writing and executing the testing protocols. We offer tailored documentation packages according to the level of validation needed by each customer.
Beginning with the End in Mind: Upfront Validation Planning
We believe in the importance of a comprehensive validation overview at the beginning of a project. Our Kickoff Validation Meeting enables us to create a plan with the customer from a cradle-to-grave perspective so that we can streamline the project work.
Quality Assurance Project Plan (QAPP)
We offer a Quality Assurance Project Plan (QAPP), which documents the validation options purchased by the customer. The QAPP outlines the deliverables for each selected option along with key resources and their responsibilities. The QAPP also details schedule milestones for the verification requirements of the project. An initial draft of the document is supplied and reviewed with the customer for comments and feedback, edited as necessary, then finalized with customer sign off.
Compliance Matrix & Functional Design Specification (FDS)
In addition, we create a Compliance Matrix, submitted with our proposal, based on the customer’s User Requirement Specification (URS). This matrix guides the development of a Functional Design Specification (FDS), which details how the mechanical and electrical designs respond to each requirement from the Compliance Matrix. The FDS also specifies the functions, operator interactions control, and sequencing associated with the equipment. This is to confirm that the proposed solution will fully meet the requirements before the equipment is built. The FDS is a living document and will be updated during the lifecycle of the project.
Reliable Code Structure and Integration
Throughout the software development process, our goal is to create a modular, testable code structure for our customers. Our engineers diligently verify their work during the design and debug phase. This ensures that the resulting code is not just functional, but also reliable with expected sequencing. The robust, reliable code can be tested both internally and by the customer, facilitating efficient and confident requirement tests.
Software Design Specification (SDS)
To keep all parties on track, we utilize a Software Design Specification (SDS) that functions as a roadmap for customers and a benchmark for our engineers. The SDS defines the principle of the architecture and configuration of the Control System software and hardware and is an outline of the software and hardware structure and configuration that will be installed for the machine.
Hardware Design Specifications (HDS)
Similarly, our Hardware Design Specifications (HDS) captures the integration of software and hardware, providing a shared roadmap for our processes. Like the SDS, it is as much a roadmap as a contract. The HDS captures the system hardware specifications and lists all the hardware components including part numbers. The HDS also describes how the machine will be connected to equipment or facility equipment and further defines the requirements for related hardware and methods of control.
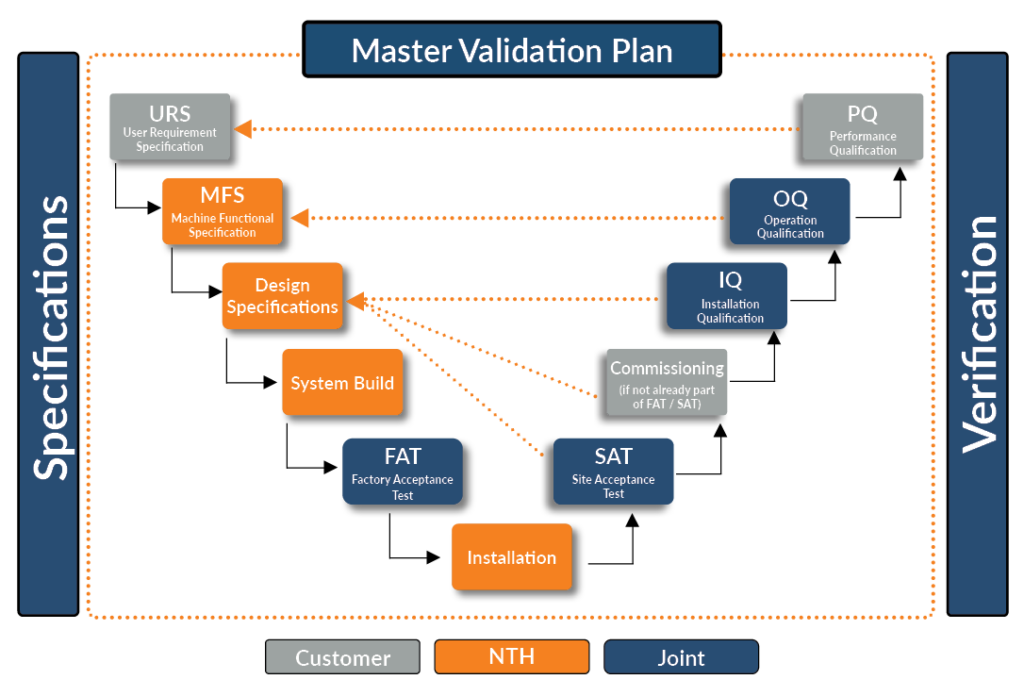
Trace Matrix
To keep track of requirement fulfillment, a Trace Matrix (Figure 1) links our Compliance Matrix with Design Specification documents. It tracks where each requirement is being met in our documentation, functioning as an itemized record for design inputs, design outputs, responsibilities, milestone, deliverables, design verifications, and design validation.
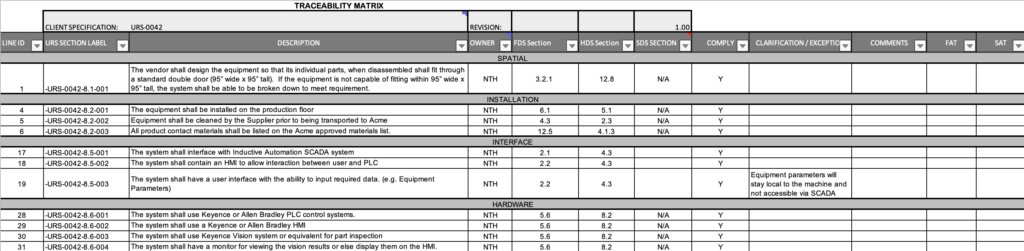
Comprehensive GAMP Documentation and Support
Automation NTH’s GAMP documentation package can also include an Installation Qualification (IQ) Test Plan and Protocol (Figure 2), and an Operation Qualification (OQ) Test Plan and Protocol.
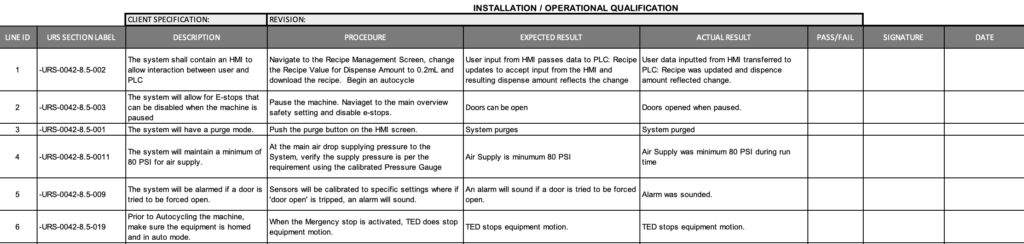
Installation Qualification (IQ)
An Installation Qualification (IQ) verifies that the system was installed and configured in accordance with the design documentation. Automation NTH writes the test protocols and then completes the execution of those test protocols to verify that the hardware components machine network configuration, circuit protection, hardware environment, software installation and backup, I/O wiring (non-inclusive) have been installed to print and meeting the intent of the design.
Installation Qualification (OQ)
Once the system has been installed and qualified by the IQ, the Operation Qualification (OQ) will verify that the system operates in accordance with the design documentation. Automation NTH can write the test protocol and then complete the execution of the test protocols to challenge the “as-built” equipment to verify that it will operate within specific limits and perform to the intent of the design, as defined in the Users Requirements Specifications (URS). This identifies and evaluates the potential failure of a process and its effect on the product’s quality. The IQ and OQ will be executed alongside the customer with NTH overseeing so that the customer has full and complete understanding of the success of the process.
Automation NTH: Your Partner in GAMP Validation
Automation NTH is committed to delivering FDA validated systems that reduce project timelines. We understand that validation can be a challenging endeavor, but with Automation NTH’s support, customers can trust in the robustness, efficiency, and reliability of their automation equipment.